![FDA Drug Recall Statistics [2024]](https://www.lightfootlawdc.com/wp-content/uploads/2024/04/fda-drug-recall-statistics-2.jpg)
The U.S. Food and Drug Administration (FDA) has the job of preserving the public health of all U.S. consumers through the regulation of a myriad of potential health risks; this includes human and veterinary drugs, biological products, medical devices, food, cosmetics, and products that emit radiation.
While the FDA’s mission is to protect consumers, many previously-approved items have resulted in recalls, long-term injuries, and lawsuits, calling into question the efficacy of their approval process.
Drugs alone have averaged 1,284 recalls per year since 2012. The continued frequency of FDA recalls has sparked both skepticism and scrutiny from the public. This comprehensive study will take a more granular look at FDA recall process since 2012 up until now.
We cover which firms experience the most recalls, how the FDA’s adverse events reporting system relates to drug recalls, and how you as a consumer can report injuries from the medical treatment you’ve been given.
Summary of Findings
- There has been a total of 15,749 drug recalls since 2012, with 15,351 recalls specifically happening in the U.S.
- Since 2012, there have been 2,193 ongoing recalls, 807 completed recalls, and 13,254 terminated recalls.
- Aidapak Services, LLC, Attix Pharmaceuticals, and King Bio Inc. are the most recalled pharmaceutical firms in the United States.
- The three states with the highest number of firms experiencing drug recalls are New Jersey (1,735), Florida (1,438), Illinois (1,266).
- There have been 22,261,316 total adverse event reports, and the frequency of adverse event reports have increased every year since 2012.
FDA Drug Recall Resources and Frequently Asked Questions
- What Is the FDA Drug Recall Process?
- FDA Drug Recall Status: Terminated, Completed, Ongoing
- The Top 100 Most Recalled Pharmaceutical Firms
- Which Country’s Pharmaceutical Firms Experience the Most Drug Recalls?
- Which U.S. State Has Firms Experiencing the Most Drug Recalls?
- What is FAERS?
- Adverse Event Statistics
- Do Drug Recalls Have to Be Issued After An Adverse Event Is Reported?
- How to Report a Drug Injury to the FDA
What Is the FDA Drug Recall Process?
According to the FDA, a drug recall is the most effective way to protect the public from a defective or potentially harmful drug. A recall is defined as a firm’s removal or correction of a distributed product that the FDA deems is in violation of its laws, but does not necessarily include a market withdrawal or stock recovery. This is because the FDA recalls drugs most commonly on a voluntary basis, and leaves it up to the responsibility of drug manufacturers and distributors to protect the public from high-risk products. Responsible firms are advised by the FDA on how to conduct an effective recall themselves.
How Are Drugs Eligible For Recall Discovered?
Most FDA drug recalls are initiated in one of the following three ways:
- A recall happens voluntarily by the product’s manufacturer or distributor.
- A company discovers that its product is defective and recalls it.
- The FDA will notify a company selling a defective drug and either suggest or (in rare, but urgent cases) request a recall.
How Does the FDA Assess the Severity of a Drug’s Risk?
In order to classify a drug recall accordingly, the FDA follows a health hazard evaluation that considers the following factors:
- Any existing diseases/injuries the product has inflicted.
- Whether any existing conditions could contribute to a larger health hazard in the future.
- An assessment of the implications the potential hazard has if it were to exist, with special attention given to the individuals who are at the greatest risk of consequences should they be exposed to the hazard.
- Assessment of the degree of seriousness the health hazard would impose on most at-risk groups.
- Assessment of the likelihood that the risk occurs.
- Assessment of the hazard’s short and long-term consequences should it occur.
Once a health hazard evaluation is conducted, the FDA categorizes the drug’s risk by class. These are referred to as Class I, Class II, and Class III, and all depend on the severity of the discovered risk:
| Class | Definition |
| Class I | A dangerous or defective product that could cause severe health complications, or even death. |
| Class II | A product with the potential to cause a temporary or serious health problem. |
| Class III | A product that is unlikely to result in any health reaction, but that violates an FDA labeling or manufacturing law. |
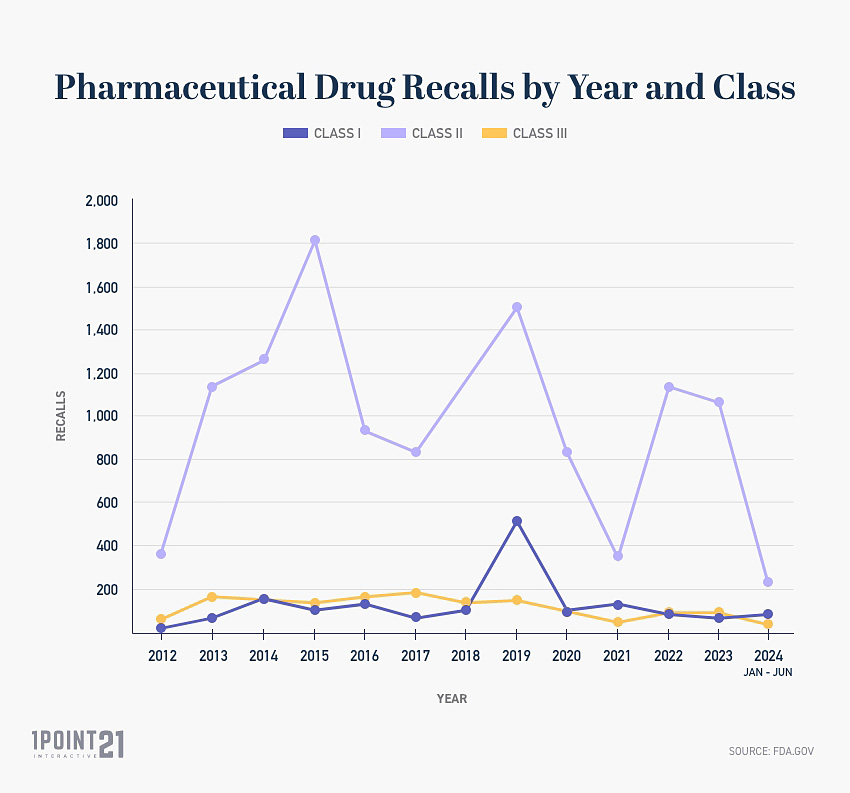
The table below details the amount of recalls per year by class and total recalls per year. [Updated for 2024]
| Year | Class I | Class II | Class III | Total |
| 2012 | 23 | 370 | 66 | 459 |
| 2013 | 67 | 1,135 | 163 | 1,365 |
| 2014 | 152 | 1,252 | 148 | 1,552 |
| 2015 | 105 | 1,812 | 135 | 2052 |
| 2016 | 130 | 934 | 167 | 1,231 |
| 2017 | 66 | 833 | 179 | 1,078 |
| 2018 | 104 | 1,155 | 146 | 1,405 |
| 2019 | 513 | 1,500 | 150 | 2,163 |
| 2020 | 110 | 829 | 100 | 1,039 |
| 2021 | 138 | 348 | 48 | 534 |
| 2022 | 83 | 1,142 | 93 | 1,318 |
| 2023 | 67 | 1,065 | 88 | 1,220 |
| 2024 (Jan-June) | 78 | 220 | 35 | 333 |
| Total | 1,636 | 12,595 | 1,518 | 15,749 |
Since 2012:
- There have been 15,749 total drug recalls issued by the FDA
- On average, 1,284 drugs are recalled every year, based upon the data from 2012 to 2023.
- 1,636 Class I drug recalls have been issued by the FDA
- 12,595 Class II drug recalls have been issued by the FDA
- 1,518 Class III drug recalls have been issued by the FDA
- Class I makes up 10.4% of all drug recalls
- Class II makes up 80% of all drug recalls
- Class III makes up 9.6% of all drug recalls
FDA Drug Recall Status: Terminated, Completed, Ongoing
While all news of FDA recalls are reported, some drugs are in different stages of determination, labelled either ongoing, completed, or terminated.
Ongoing Recalls: A recall that is in the process of taking the necessary safety precautions advised by the FDA.
Completed Recalls: A recall that reaches the point at which the firm has retrieved and captured all outstanding product that could reasonably be expected to be recovered, or has completed all product corrections advised by the FDA.
Terminated Recalls: A recall where the FDA has determined that all reasonable efforts have been made to remove or correct the violative product to the best of their ability.
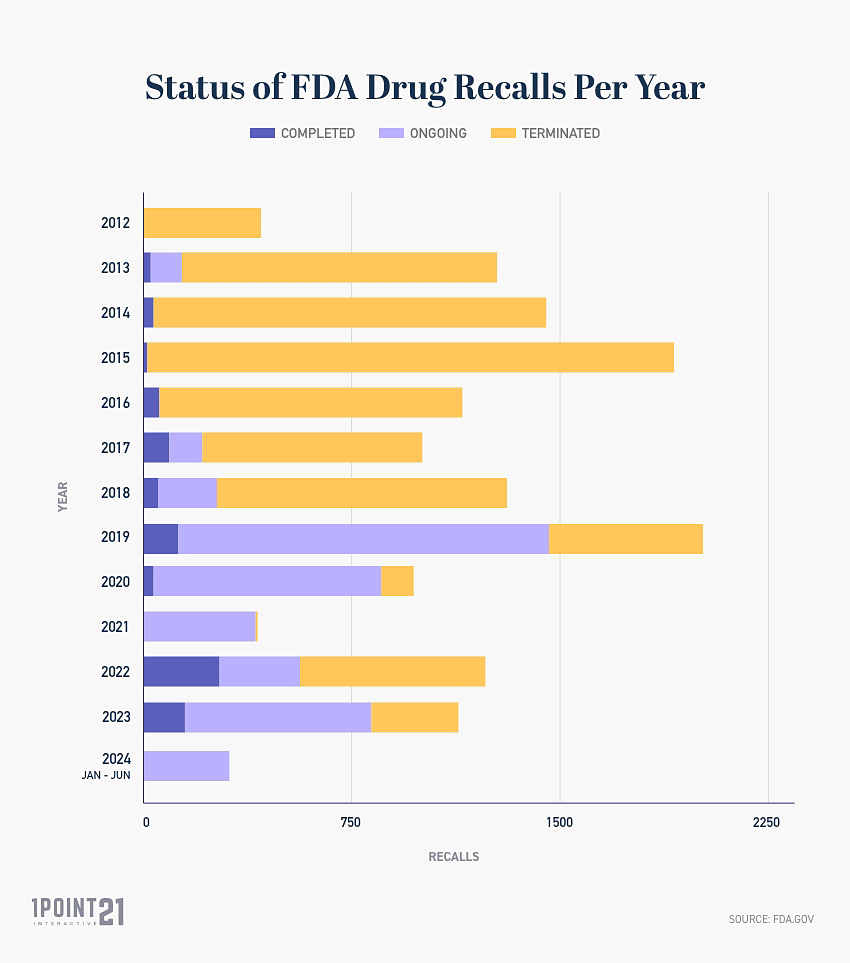
What Is the Status of All FDA Recalled Drugs?
Between 2012 and June 2024, the FDA drug recall statuses are:
Ongoing Recalls: 2,193
Completed Recalls: 807
Terminated Recalls: 13,254
Status of FDA Drug Recalls Per Year
The table below highlights the status of FDA drug recalls per year. The data is updated as of June 2024.
| Classification Year | Status | Count of Status |
| 2012 | Ongoing | 1 |
| 2012 | Terminated | 458 |
| 2013 | Completed | 29 |
| 2013 | Ongoing | 118 |
| 2013 | Terminated | 1218 |
| 2014 | Ongoing | 36 |
| 2014 | Terminated | 1516 |
| 2015 | Completed | 1 |
| 2015 | Ongoing | 8 |
| 2015 | Terminated | 2043 |
| 2016 | Ongoing | 57 |
| 2016 | Terminated | 1174 |
| 2017 | Completed | 100 |
| 2017 | Ongoing | 126 |
| 2017 | Terminated | 852 |
| 2018 | Completed | 52 |
| 2018 | Ongoing | 233 |
| 2018 | Terminated | 1120 |
| 2019 | Completed | 132 |
| 2019 | Ongoing | 1430 |
| 2019 | Terminated | 601 |
| 2020 | Completed | 32 |
| 2020 | Ongoing | 885 |
| 2020 | Terminated | 122 |
| 2021 | Completed | 4 |
| 2021 | Ongoing | 433 |
| 2021 | Terminated | 6 |
| 2022 | Completed | 293 |
| 2022 | Ongoing | 310 |
| 2022 | Terminated | 718 |
| 2023 | Completed | 160 |
| 2023 | Ongoing | 724 |
| 2023 | Terminated | 336 |
| 2024 | Completed | 1 |
| 2024 | Ongoing | 331 |
| 2024 | Terminated | 1 |
This information serves as an indication of how long the process can take for an FDA drug recall to be officially terminated, demonstrated by 2012 and 2013 drug recalls remaining in the ongoing/completed phase.
The Most Recalled Pharmaceutical Firms
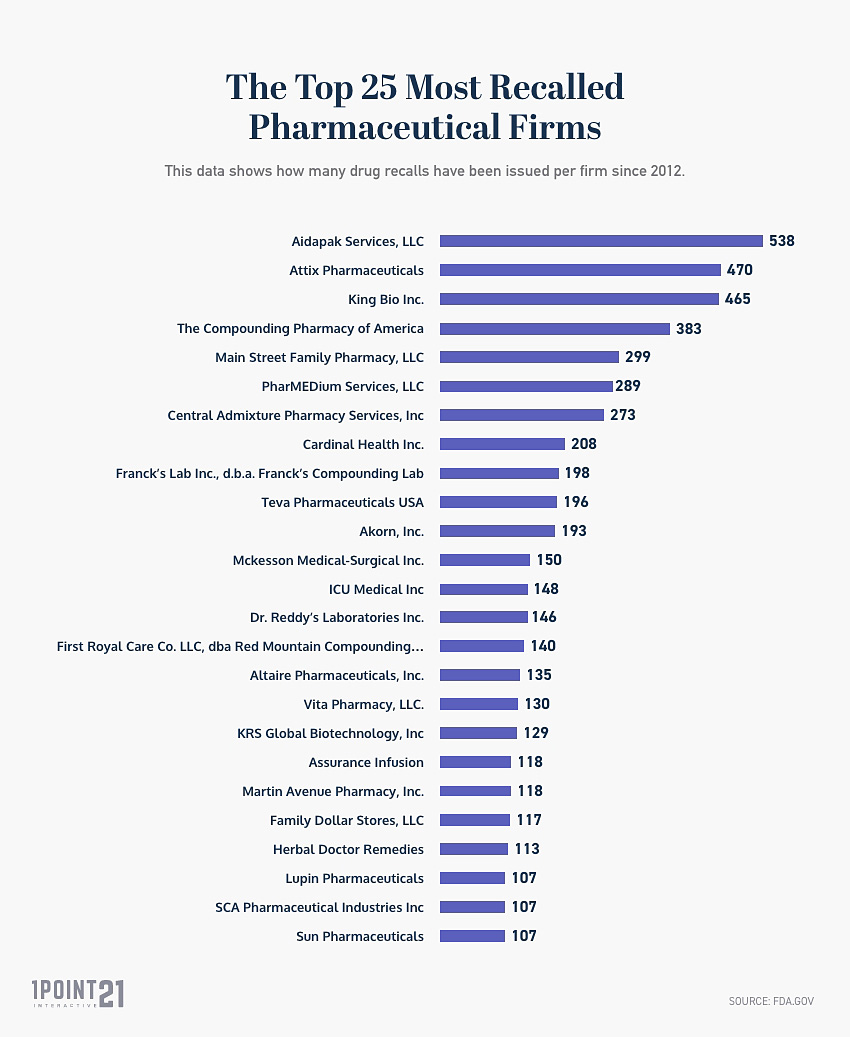
This data shows how many drug recalls have been issued per firm since 2012. The data below is updated as of June 2024.
| Recalling Firm Name | Amount of Recalls |
| Aidapak Services, LLC | 538 |
| Attix Pharmaceuticals | 470 |
| King Bio Inc. | 465 |
| The Compounding Pharmacy of America | 383 |
| Main Street Family Pharmacy, LLC | 299 |
| PharMEDium Services, LLC | 289 |
| Central Admixture Pharmacy Services, Inc | 273 |
| Cardinal Health Inc. | 208 |
| Franck’s Lab Inc., d.b.a. Franck’s Compounding Lab | 198 |
| Teva Pharmaceuticals USA | 196 |
| Akorn, Inc. | 193 |
| Mckesson Medical-Surgical Inc. | 150 |
| ICU Medical Inc | 148 |
| Dr. Reddy’s Laboratories Inc. | 146 |
| First Royal Care Co. LLC, dba Red Mountain Compounding Pharmacy. | 140 |
| Altaire Pharmaceuticals, Inc. | 135 |
| Vita Pharmacy, LLC. | 130 |
| KRS Global Biotechnology, Inc | 129 |
| Assurance Infusion | 118 |
| Martin Avenue Pharmacy, Inc. | 118 |
| Family Dollar Stores, LLC | 117 |
| Herbal Doctor Remedies | 113 |
| Lupin Pharmaceuticals | 107 |
| SCA Pharmaceutical Industries Inc | 107 |
| Sun Pharmaceuticals | 107 |
| Pfizer Inc. | 106 |
| Lowlite Investments, Inc. D/B/A Olympia Pharmacy | 105 |
| Perigo Company PLC | 104 |
| JD & SN Inc., dba Moses Lake Professional Pharmacy | 101 |
| Pharm D Solutions, LLC | 100 |
| Advanced Pharma Inc. | 99 |
| Wells Pharmacy Network LLC | 97 |
| Medaus, Inc. | 96 |
| Olympia Compounding Pharmacy dba Olympia Pharmacy | 94 |
| Western Drug | 91 |
| Pharmakon Pharmaceuticals, Inc. | 89 |
| RXQ Compounding LLC | 88 |
| Beacon Hill Medical Pharmacy, P.C. | 86 |
| Synergy Rx | 81 |
| Tri-Coast Pharmacy | 80 |
| Key Pharmacy and Compounding Center | 79 |
| FVS Holdings, Inc. dba. Green Valley Drugs | 78 |
| Infusion Options, Inc. | 76 |
| Accord Healthcare, Inc | 75 |
| Medline Industries Inc | 75 |
| Washington Homeopathic Products, Inc. | 75 |
| Nephron Sterile Compounding Center, LLC | 72 |
| American Pharmaceutical Ingredients LLC | 71 |
| Lupin Pharmaceuticals Inc. | 71 |
| Mylan Pharmaceuticals Inc. | 70 |
| Reliable Rexall-A Compounding Pharmacy | 70 |
| Walgreens Infusion Services | 69 |
| Anderson Compounding Pharmacy, Inc. DBA Anderson Compounding Pharmacy | 68 |
| Kroger Specialty Pharmacy, Inc. | 67 |
| Sage Products Inc | 66 |
| Ultra Seal Corporation | 66 |
| Health Innovations Pharmacy, Inc | 65 |
| US Compounding Inc | 65 |
| Fusion IV Pharmaceuticals, Inc. dba Axia Pharmaceutical | 64 |
| Teligent Pharma, Inc | 63 |
| Edge Pharma, LLC | 62 |
| American Health Packaging | 59 |
| Pharmacy Innovations | 59 |
| Oregon Compounding Centers, Inc. dba Creative Compounds | 56 |
| The Medicine Shoppe Pharmacy | 55 |
| Lincare, Inc. | 54 |
| Product Quest Manufacturing LLC | 54 |
| Aurobindo Pharma USA Inc | 53 |
| Sentara Fusion Services | 53 |
| Astral SteriTech Private Ltd. | 52 |
| Baxter Healthcare Corp. | 52 |
| Cantrell Drug Company | 52 |
| Promise Pharmacy, LLC | 52 |
| RemedyRepack Inc. | 52 |
| TMC Acquisition LLC. | 52 |
| Specialty Medicine Compounding Pharmacy | 51 |
| Well Care Compounding Pharmacy | 51 |
| Avella of Deer Valley, Inc. Store 38 | 50 |
| Clinical Specialties Compounding Pharmacy | 50 |
| Partell Specialty Pharmacy | 49 |
| Downing Labs, LLC | 48 |
| Glenmark Pharmaceuticals Inc., USA | 48 |
| Natures Pharmacy & Compounding Center | 48 |
| Novartis Consumer Health | 48 |
| the Compounder | 47 |
| Golden State Medical | 45 |
| Wockhardt Usa Inc. | 45 |
| Fresenius Kabi USA, LLC | 45 |
| TG United, Inc. | 44 |
| Torrent Pharma Inc | 43 |
| Abbott’s Compounding Pharmacy, Inc. | 43 |
| Cardinal Health | 43 |
| Nora Apothecary and Alternative Therapies, Inc. | 43 |
| Apotex Inc. | 41 |
| Preferred Pharmaceuticals | 41 |
| Zydus Pharmaceuticals USA Inc | 41 |
| Kalman Health & Wellness, Inc. dba Essential Wellness Pharma | 39 |
| Baptist Health Medical Towers Pharmacy and Infusion Services | 38 |
| Torrent Pharma Inc. | 38 |
| Baxter Healthcare Corporation | 37 |
| AuroMedics Pharma LLC | 36 |
| Lloyd Inc. of Iowa | 36 |
| Michigan Herbal Remedies | 36 |
| NingBo Huize Commodity Co., Ltd | 36 |
| MICHIGAN HERBAL REMEDIES | 36 |
| NingBo Huize Commodity Co.,Ltd. | 36 |
| SterRx, LLC | 35 |
| Taro Pharmaceuticals | 35 |
| The Harvard Drug Group | 35 |
| Colonia Care Pharmacy | 34 |
| Sentara Enterprises | 34 |
| ULTRAtab Laboratories, Inc | 34 |
| AVKARE Inc. | 33 |
| QuVa Pharma, Inc. | 33 |
| Teva Pharmaceuticals USA, Inc. | 33 |
| AuroMedics Pharma LLC | 32 |
| CMC Enterprise Pharmacy | 32 |
| Hartley Medical Center Pharmacy, Incorporated | 32 |
| Direct Rx | 32 |
| QuVa Pharma, Inc. | 32 |
| VistaPharm, Inc. | 32 |
| Coast Quality Pharmacy, LLC dba Anazao Health | 31 |
| The Apothecary Shoppe LLC | 31 |
| Franck’s Lab Inc dba Trinity Care Solutions | 30 |
| Jubilant Cadista Pharmaceuticals, Inc. | 29 |
| Macleods Pharma Usa Inc. | 28 |
| MasterPharm LLC | 28 |
| Pharmakon Pharmaceuticals | 28 |
| West-Ward Pharmaceutical Corp. | 28 |
| Carefusion 213, LLC | 27 |
| Noven Pharmaceuticals, Inc. | 27 |
- Aidapak Services, LLC has been the most highly recalled firm since 2012.
- Located in Vancouver, WA
- 538 recalled drugs
- Firm has only 10 employees
- Plastic Resin & Synthetic Fiber Manufacturing Industry
- Firm averages $1.72 million annually
- The top 3 most FDA recalled firms are Aidapak Services, LLC (538), Attix Pharmaceuticals (470), and King Bio Inc (465).
- Pfizer is the 26th most highly recalled firm by the FDA.
Which Country’s Pharmaceutical Firms Experience the Most Drug Recalls?
| Recalling Firm Country | Count of Recalling Firm Country |
| United States | 15,351 |
| Canada | 560 |
| India | 156 |
| China | 63 |
| Mexico | 43 |
| Jordan | 17 |
| Germany | 15 |
| Spain | 13 |
| Guyana | 7 |
| Turkey | 6 |
| Guatemala | 5 |
| United Arab Emirates | 5 |
| Japan | 4 |
| Israel | 2 |
| Switzerland | 3 |
| Aruba | 2 |
| United Kingdom | 1 |
| Dominican Republic | 1 |
- The FDA has issued 15,351 drug recalls in the U.S. since 2012
- 94% of FDA drug recalls have been in the United States
- 4% of FDA drug recalls have been in Canada
Which U.S. State Has Firms Experiencing the Most Drug Recalls?
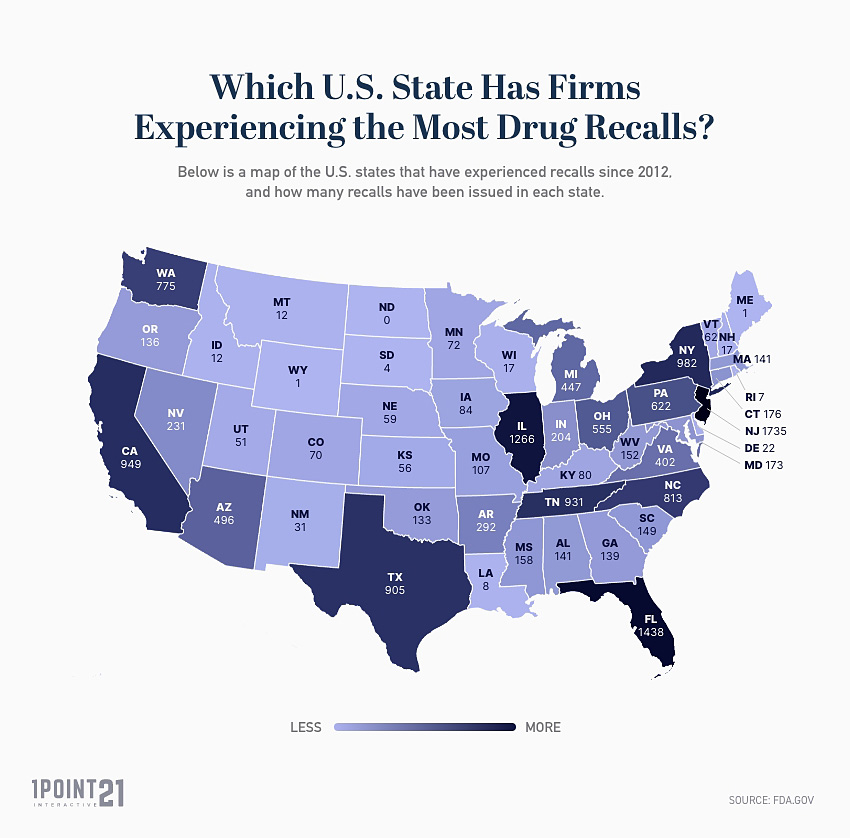
Below is a list of the U.S. states that have experienced recalls since 2012, and how many recalls have been issued in each state:
| State | Number of Recalls |
| New Jersey | 1735 |
| Florida | 1438 |
| Illinois | 1266 |
| New York | 982 |
| California | 949 |
| Tennessee | 931 |
| Texas | 905 |
| North Carolina | 813 |
| Washington | 775 |
| Pennsylvania | 622 |
| Ohio | 555 |
| Arizona | 496 |
| Michigan | 447 |
| Virginia | 402 |
| Arkansas | 292 |
| Nevada | 231 |
| Indiana | 204 |
| Connecticut | 176 |
| Maryland | 173 |
| Mississippi | 158 |
| West Virginia | 152 |
| South Carolina | 149 |
| Alabama | 141 |
| Massachusetts | 141 |
| Georgia | 139 |
| Oregon | 136 |
| Oklahoma | 133 |
| Missouri | 107 |
| Iowa | 84 |
| Kentucky | 80 |
| Minnesota | 72 |
| Colorado | 70 |
| Vermont | 62 |
| Nebraska | 59 |
| Kansas | 56 |
| Utah | 51 |
| New Mexico | 31 |
| Delaware | 22 |
| New Hampshire | 17 |
| Wisconsin | 17 |
| Idaho | 12 |
| Montana | 12 |
| Louisiana | 8 |
| Rhode Island | 7 |
| South Dakota | 4 |
| Maine | 1 |
| Wyoming | 1 |
| Alaska | 0 |
| Hawaii | 0 |
| North Dakota | 0 |
- New Jersey is the U.S. state with the leading number of firms experiencing recalls, with 1,735 FDA recalls issued since 2012.
- The 3 states with highest number of firms experiencing drug recalls are New Jersey (1,735), Florida (1,438), and Illinois (1,266).
What is FAERS?
The FDA Adverse Events Reporting System (FAERS) Public Dashboard, is an online database created by the FDA in order to query adverse event reports in an easily accessible way for the general public. All information in the dashboard has been reported to the FDA by pharmaceutical companies, healthcare providers, and consumers themselves. FAERS reports include both drug and biologic product injuries. The public dashboard categorizes adverse event reports by report type: Direct, Mandatory, Expedited, and Non-Expedited:
| Report Type | Definition |
| Direct | Voluntarily submitted reports to the FDA through the MedWatch program by consumers and healthcare workers. |
| Mandatory | Reports submitted by manufacturers, and categorized as either expedited or non-expedited. |
| Expedited | Contains at least one adverse event that is not currently disclosed in the labeling and causes a serious outcome on the user. |
| Non-Expedited | Do not meet criteria for expedited reports, but include reports that are either serious and expected, non-serious and unexpected, or non-serious and expected. |
What Is an Adverse Event?
An adverse event is any event that causes unintended and sometimes harmful occurrences to a patient as a result of the medical treatment they received. An occurrence still qualifies as an adverse event whether it was preventable or nonpreventable, intended or unintentional. According to the CDC, adverse drug events cause around 1.3 million emergency department visits per year, and about 350,000 of those patients need to be hospitalized for further treatment after the drug event occurs.
What Is an Example of an Adverse Drug Reaction?
Examples of adverse events include any symptoms or diseases associated with a patient’s medical care. Some common examples of adverse drug reactions include:
- Rashes
- Allergic reactions
- Kidney damage
- Anemia
- Nerve injuries
- Overdoses
Adverse Event Statistics
All the below adverse event data includes reports from 2012 until June 2024.
- There have been 22,261,316 total adverse event reports.
- There have been 12,448,889 expedited adverse event reports.
- There have been 9,059,767 non-expedited adverse event reports.
- There have been 752,660 direct adverse event reports.
- There have been 11,779,833 serious reports (excluding death).
- There have been 1,935,894 death reports.
- Frequency of adverse event reports have increased every year since 2012.
Do Drug Recalls Have to Be Issued After An Adverse Event Is Reported?
According to FAERS, not all reports of injuries result in a recall. The mission the FDA’s adverse events reporting system has is to make the FDA aware of the severity of the drug reaction, initiate an assessment of its short and long-term implications, and notify manufacturers, distributors and consumers of its findings. While this sometimes results in a recall, it more commonly results in one of the following actions:
- Updating a product’s labeling information
- Restricting the use of the drug
- Communicating new safety information to the public
How Can I Report a Drug Injury to the FDA?
Anyone is able to report an adverse event using the FDA’s MedWatch Voluntary Reporting Program. Reporting is essential for keeping track of a drug’s behavior and contributes to keeping the FDA, pharmaceutical companies, and general public informed. If you’ve been injured by a drug, a Washington D.C. drug injury lawyer can help you recover.
Citations
- https://datadashboard.fda.gov/ora/cd/recalls.htm
- https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis
- https://www.fda.gov/drugs/drug-recalls/fdas-role-drug-recalls
- https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard
- https://www.accessdata.fda.gov/scripts/medwatch/index.cfm